Chiroteuthis
Clyde F. E. Roper, Richard E. Young, and Michael Vecchione

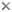
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Species of Chiroteuthis are the most commonly encountered species of the family. They possess a variety of photophores that can produce some unusual bioluminescent displays. The title photograph shows the series of photophores along the ventral arms, the rows of photophores on the ventral surface of the eyes (the arrangement of ocular photophores, however, varies with the species) and the visceral photophores (absent in C. mega). The tentacles carry a series of complex, peculiarly constructed, pad-like photophores along the tentacular stalk that distally are mostly embedded in the aboral surface of the club. The last member of this photophore series, the club-tip photophore, however, is greatly enlarged and possesses muscular lids that open and close over the organ. The function(s) of these photophores are unknown although the club-tip photophore could act as a lure to attract prey (Voss, 1956). The very long tentacles can be completely retracted into elongate tubes formed by the broad lateral membranes (=tentacular sheath) of the fourth arms. Arms IV (the ventral arms) are not only much longer but, also, thicker than the other arms. The tentacular clubs are distinctive at the generic level and often at the specific level. The large fourth arms contain many large vesicles that hold ammonium chloride. This low-density fluid apparently, is responsible for the typical oblique orientation of the squid with the arms uppermost, as observed from submersibles and as shown in the title photograph on the family page.


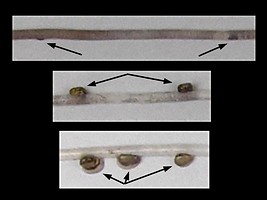
Figure. Chiroteuthis aff. veranyi, 75 mm ML, off New Zealand. Left - Ventral view. Right - Enlarged views of the three boxes on the upper tentacle in the left image showing the decreasing size, increasing spacing of the tentacle photophores with distance on the tentacles and the virtually embedded, more distal photophores. Photographs by Darren Stevens, NIWA.
Diagnosis
A chiroteuthid ...
- with suckers on both proximal and distal regions of club.
- with typical tragus and antitragus on funnel locking-apparatus.
- with a row of photophores along each arm IV.
Characteristics
- Arms
- Arms IV much the longest and greatly thickened.
- Arms IV much the longest and greatly thickened.
- Tentacles
- Suckers in four series throughout club.
- Suckers in four series throughout club.
- Head
- Olfactory organ located on stalk just posterior to each eye.
- Olfactory organ located on stalk just posterior to each eye.
- Funnel
- Funnel valve present.
- Locking-apparatus oval with typical tragus and antitragus.
- Photophores
- A series of photogenic spheres along arms IV (each photophore alternates with a lateral arm sucker). These photophores look silvery (see title photograph) or red when covered by chromatophores (see below).
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window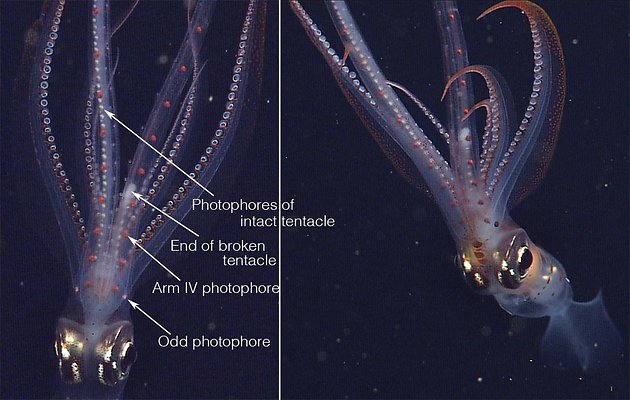
Figure. Two photographs of the same subadult C. calyx from slightly different views showing the photophores of Arms IV (red) and those of the tentacular stalk (silvery). Left - Ventral view of the head and arms. Note that one tentacle is mostly missing allowing easy recognition of the red photophores of Arms IV and the silvery photophores of the highly contracted tentacles enclosed in the lateral membranes (= tentacular sheath) of arms IV. Right - Anterior oblique view of nearly the entire squid. Note the "Odd photophore," not previously recognized, that is not in exact alignment with the rest of the Arm IV photophores. Photographs, taken in situ with an MBARI ROV at 400 m depth, © 2013 MBARI.
- Visceral photophores present in all but one species.
- Ocular photophores present as stripes or series of round organs or combination of both.
- Pad-like photophores present on tentacular stalk, distally embedded in aboral surface of club.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window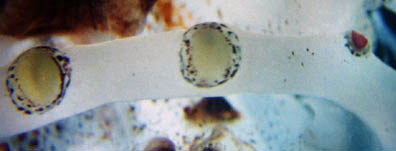
Figure. View of three pad-like tentacle-stalk photophores of C. picteti. Note that one is small and has a violet pigmentation. We do not know if this is normal or a result of loss and regeneration. See also the pad-like photophores of Asperoteuthis. Photograph by R. Young.
- Large club-tip photophore.
- Numerous small photophores on oral surface of distal tentacular stalk.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new windowFigure. Oral view of the tentacle-stalk at base of the club of Chiroteuthis sp., Hawaiian waters. The arrow points to one of many small, light-colored photophores present on the stalk but often difficult to detect.
- A series of photogenic spheres along arms IV (each photophore alternates with a lateral arm sucker). These photophores look silvery (see title photograph) or red when covered by chromatophores (see below).
Discussion of Phylogenetic Relationships
Within Chiroteuthis, the species can be grouped provisionally on the basis of the following characters.C. picteti group: C. picteti, C. mega
- Ocular photophores in three series/stripes on the eyeball.
- Clubs long with protective membranes in two sections with length:length ratios of > 1:8.
- Distal section with broad, triangular, closely-spaced trabeculae.
- All pigment on clubs in chromatophore organs rather than epithelial cells.
- One large central tooth on club sucker rings.
C. joubini group: C. joubini, C. spoeli, C. sp. B2.
- Ocular photophores in two series on the eyeball.
- Clubs short with protective membranes in three sections.
- Distal section with narrow, broadly spaced trabeculae.
- Much pigment on clubs in epithelial cells rather than chromatophores.
- No large central tooth on club sucker rings.
C. veranyi group: C. veranyi, C. aff. veranyi, C. calyx
- Ocular photophores in two stripes and several round photophores.
- Club short with protective membranes in two, nearly equal, sections.
- One large central tooth on club sucker rings.

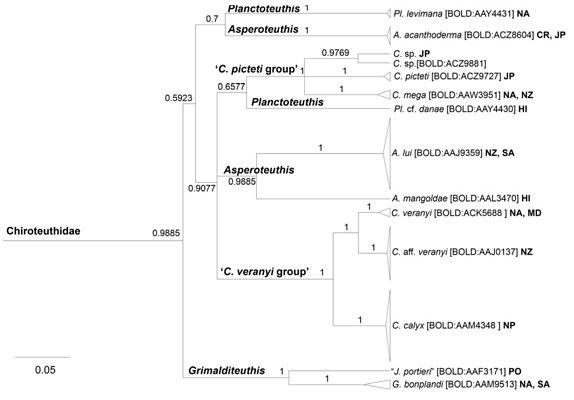
Figure. Excerpt from a combined Bayesian phylogeny of all the chiroteuthid family specimens sequenced by Braid et al. (2017) and previously published sequences for COI, 12S rRNA, and 16S rRNA. Node values indicate posterior probabilities, and branches with values lower than 0.5 have been collapsed. Barcode Index Numbers (BINs) are included beside species names (in square brackets). Clades are identified to the highest taxonomic level. The following abbreviations are used to indicate locality data: Caribbean (CR); Hawaii (HI); Japan (JP); Mediterranean (MD); North Atlantic (NA); North Pacific (NP); New Zealand (NZ); Pacific Ocean (PO); South Atlantic (SA); Southern Ocean (SO). Modified from Braid et al. (2017) Fig. 3. © 2017
Speciation in Chiroteuthis
Within each of the three groups there exists considerable unexplained variation. Our knowledge of speciation in this group is minimal. Future detailed evaluation of the variation could indicate that some of the species recognized above will be synonymized (e.g., all forms in the C. joubini group could represent a single, highly variable species) or that additional new species will be recognized within the forms now known (e.g., see the C. picteti group in the above phylogeny chart). We caution anyone against naming species in this genus until a better understanding of the variability is known.
Life History
The developing adult club in the doratopsis
The late doratopsis stages of the paralarval period have tentacles that possess a distal, expanded, paralarval club and a proximal presumptive (developing) adult club. The latter has four series of very small suckers which are more regularly arranged and extend over a much longer region of the tentacular stalk than in paralarvae of the other genera except New Genus B.


Figure. Oral view of a tentacle from an unidentified 28 mm ML doratopsis. The paralarval club has large suckers and a keel (but without division into manus and dactylus); the developing adult club on the tentacular stalk has small suckers. Drawing from Roper and Young, 1967.
Decorated tail of the doratopsis
The tail of the late doratopsis stage in Chiroteuthis is highly decorated ( as in Planctoteuthis) but is lost at the end of this stage. We suspect that the decorations of the tail may be species-specific but details of the decorations of the tail in most species are unknown. MBARI researchers, however, have gotten some excellent photographs of the tail of Chiroteuthis calyx, some of which are seen below.
All members of the family lack hectocotylized arms. Species of Chiroteuthis, at least, have an elongate penis that can extend beyond the mantle opening and probably compensates for the absence of a hectocotylus.

Figure. Ventral view of the viscera of a mature male Chiroteuthis spoeli with the long, slender penis extending to the right. A relatively small gill lies beneath the penis. The testis is white as are the spermatophores within Needham's sac to the right of the testis. The insert to the right shows the penis at a different angle where the broad, flat tip is more apparent. Also seen are the large visceral photophores and the violet renal appendages.
Behavior
Occasionally species of Chiroteuthis are observed, in mid-depths, from an submersible or ROV with their long arms IV extended horizontally and their tentacles hanging vertically downward from the tips of the tentacular sheaths of arms IV (see title photograph above). The assumption is that the squid is "fishing," i.e., waiting for prey to be caught by the dangling tentacular clubs (e.g. Voss, 1956). Recently this behavior also was, surprisingly, observed very near the deep ocean floor. Here is what was seen (begin at the right):

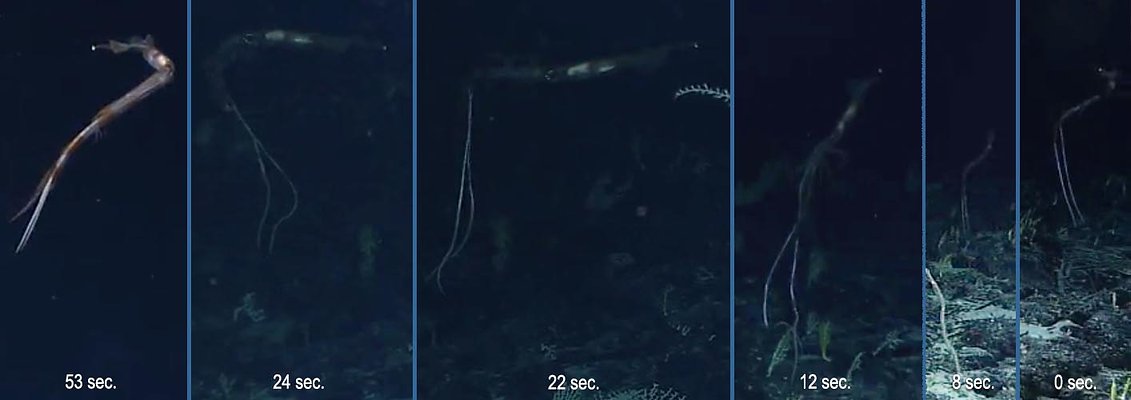
Figure. Chiroteuthis sp. Video frames from an ROV operated from NOAA Ship OKEANOS EXPLORER, north of Samoa; bottom depth 1105 m.
0 sec.: The ROV observers note an object in the distance (the object proves to be a squid with dangling tentacles) moving slowly just above the ocean floor.
8 sec.: The camera has zoomed-out and panned. The squid continues to over the ocean floor in much the same attitude with tentacles trailing downward from the ventral arms.
12 sec.: The camera zooms-in and, presumably, lights and ROV start to approach squid. The squid is clearly disturbed, its attitude has changed and the tentacles begin to move.
22 sec.: The squid is horizontal to the bottom and moving away.
24 sec.: The squid is moving forward at a faster pace and the ventral arms and tentacles are swept backward. The squid then moves out of clear vision of the camera. and the ROV pursues.
53 sec.: The ROV approaches the hovering squid well off the ocean floor and the tentacles (white lines) are nearly fully retracted.
Below are subsequent photos taken of the squid. These show (1) the best views of the eye photophores and (2) the peculiar display of banding on the arms, head and mantle of the squid. We are uncertain if there are two or three series of photophores on the eye; therefore, the squid is identified only as Chiroteuthis sp. The white spot at the posterior tip of the gladius is, apparently, an injury that was detectable when the squid first came into view.
Distribution
Within two of the species groups, geographical distributions appear to be non-overlapping although the data records are few for all species.
In the C. veranyi group, C. veranyi is known from the Atlantic and Indian Oceans and supposedly in the Pacific Ocean from the South and Equatorial Pacific (although C. "veranyi" specimens from New Zealand were recently sequenced and proved distinct from C. veranyi material from other regions; see the C. aff. veranyi page). C. calyx is known only from the temperate and boreal North Pacific.
In the C. picteti group, C. picteti is known from the tropical and subtropical Indo-eastern Pacific while C. mega is known from the Atlantic Ocean, the western Pacific and the temperate South Pacific waters off New Zealand.
In contrast different distribution patterns do not separate all members of the C. joubiniChiroteuthis spoeli group. is known from the tropical Pacific and the tropical south Atlantic to the temperate North Atlantic and the western Indian Ocean. Chiroteuthis. sp. B2 is known from the subtropical and temperate South Atlantic. C. joubini is known from the subtropical North and temperate South Atlantic.
References
Braid, H. E., Kubodera, T., & Bolstad, K. S. (2017). One step closer to understanding the chiroteuthid families in the Pacific Ocean. Marine Biodiversity, 47(3), 659-683.
Chun, C. 1910. Die Cephalopoden. Oegopsida. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition, "Valdivia" 1898-1899, 18: 1-522 + Atlas.
Hunt, J. C. 1996. The behavior and ecology of midwater cephalopods from Monterey Bay: Submersible and laboratory observations. Ph. D. Dissertation, Univ. Calif. Los Angeles. 231 pp.
Vecchione, M., B. H. Robison, and C. F.E. Roper. 1992. A tale of two species: tail morphology in paralarval Chiroteuthis (Cephalopoda: Chiroteuthidae). Proceeding of the Biological Society of Washington 105(4): 683-692.
Roper, C. F. E. and R. E. Young (1967). A review of the Valbyteuthidae and an evaluation of its relationship with the Chiroteuthidae. Proc. U.S. Nat. Mus., 123: 1-9.
Voss, G. L. 1956. Review of the cephalopods of the Gulf of Mexico. Bull. Mar. Sci. Gulf Carib. 6: 85-178.
Young, R. E. (1991). Chiroteuthid and related paralarvae from Hawaiian waters. Bull. Mar. Sci., 49: 162-185.
Information on the Internet
Sequences used in the chiroteuthid families phylogeny (Braid et al., 2017) are available on the Barcode of Life Database (BOLD), in a public project titled "Chiroteuthid Families" (project code: CHSQX).Title Illustrations

| Scientific Name | Chiroteuthis calyx |
|---|---|
| Location | Monterey Bay Canyon, Northeast Pacific at 36.7°N, 122.0°W. |
| Comments | Image courtesy of the Monterey Bay Aquarium Research Institute (MBARI). You must obtain permission from MBARI to use this photo; please contact pressroom@mbari.org for further information |
| Acknowledgements | Susan Von Thun, photo editing, MBARI |
| Specimen Condition | Live Specimen |
| Behavior | Photograph shows the tentacles being extended (or retracted) from the tentacular sheaths formed by the lateral membranes of the elongate arms IV. |
| View | Side |
| Copyright | © 2011 MBARI |
About This Page

Smithsonian Institution, Washington, D. C., USA

University of Hawaii, Honolulu, HI, USA

National Museum of Natural History, Washington, D. C. , USA
Page copyright © 2019 , , and
 Page: Tree of Life
Chiroteuthis .
Authored by
Clyde F. E. Roper, Richard E. Young, and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Chiroteuthis .
Authored by
Clyde F. E. Roper, Richard E. Young, and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 26 March 2019
Citing this page:
Roper, Clyde F. E., Richard E. Young, and Michael Vecchione. 2019. Chiroteuthis . Version 26 March 2019 (under construction). http://tolweb.org/Chiroteuthis/19462/2019.03.26 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site