Hymenochaetales
Hymenochaetoid clade
Hymenochaetaceae
Aristóteles Góes-Neto and Cláudia Groposo

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The hymenochaetoid clade is one of the eight major clades of Homobasidiomycetes suggested by Hibbett and Thorn (2001), and corroborated by Binder and Hibbett (2002) and Moncalvo et al. (2002). The hymenochaetoid clade probably comprises all known Hymenochaetaceae, as well as some members of at least nine different families of Homobasidiomycetes, as revealed by the aforementioned recent molecular phylogenetic studies.
Although the Hymenochaetaceae are typically represented by the yellowish to reddish brown lignicolous bracket fungi, the non-hymenochaetacean species of the hymenochaetoid clade exhibit a striking diversity of forms, colours and habits, which includes mushrooms (Fig. 1), lilac to violet bracket fungi (Fig. 2), some members of Corticiaceae s.l. that resemble a splash of paint on trunks and twigs of trees (Fig. 3), and even one species that was for a long time in the "Guinness Book of World Records" as possessing the largest known fruiting body of a fungus, Bridgeoporus nobilissimus (Fig. 4), a rare and endangered species, restricted to very large specimens of noble firs in Pacific Northwest old-growth forests in North America (Burdsall et al., 1996).

Clockwise from top left: Figure 1: Salmon basidiomata of Loreleia marchantiae (Singer & Clémençon) Redhead, Moncalvo, Vilgalys & Lutzoni on the liverwort Marchantia. © Cathy L. Cripps. Figure 2: Lilac basidiome of Trichaptum byssogenum (Jungh.) Ryv. on dead tree trunk. © Aristóteles Góes-Neto. Figure 3: Basidiome of Hyphodontia sambuci (Pers.) J. Erikss. on tree trunk. © Paul Busselen. Figure 4: Basidiomata of Bridgeoporus nobilissimus (W.B. Cooke) Volk, Burdsall, & Ammirati. © Tom Volk.
Many species of the hymenochaetoid clade, especially Phellinus s.l. are important phytopathogens, causing white rot in living trees in natural and cultivated forests of both conifers and flowering plants (Larsen and Cobb-Poule, 1990; Ryvarden, 1991, 1993): Phellinus pini is the etiological agent of red ring rot, the most economically important of the stem rotters of pines (Harrington and Wingfield, 1998); Phellinus weirii causes the laminated root rot in Douglas-fir and other commercially important species of conifers in northwestern North America (Thies and Rona, 1995); and Fomitiporia punctata and Fomitiporia mediterranea are associated with "esca" disease of grapevines (Fischer, 2002). On the other hand, some representatives of the hymenochaetoid clade have been used for medicinal purposes by traditional human societies in Asia (Lim et al., 2003.), and many metabolic compounds isolated from them have antineoplasic and antioxidant activities (Ajith and Janardhanan, 2003; Shon et al., 2003). Furthermore, because of their ability of degrading natural recalcitrant molecules (e. g. liginin, the main component of wood) they have been studied to be used for the biodegradation of xenobiotics (non-natural, man-made compounds, e. g. plastics, biocides etc.) (Wesenberg et al., 2003).
Characteristics
Except for only one common cytological character, this clade apparently has no morphological, physiological or ecological synapomorphy, and it is almost exclusively defined on molecular grounds. Although not confirmed for all the studied species regarded as members of the hymenochaetoid clade, they all appear to share at least one nonmolecular character in common, the dolipore septa with imperforate parenthesomes (Moore, 1985; Oberwinkler, 1985; Langer and Oberwinkler, 1993; Keller, 1997; Muller et al., 2000, see Characteristics of Hymenomycetes).
The Hymenochaetaceae are rather well-defined. They always possess clampless generative hyphae (Fig. 6) and brownish basidiomata (Fig. 5) that darken when moistened in alkali solutions (permanent positive xanthochroic reaction). Setoid structures, especially unbranched setae (Figs. 7-8), but also branched asterosetae occur frequently in several taxa (Oberwinkler, 1977). However, the hymenochaetoid species assigned to the other families not only lack this combination of characters but also have other markedly different nonmolecular characters.

Top: Figure 5: Browinsh basidiome of Phellinus palmicola (Berk. & Curt.) Ryv. Bottom left to right: Figure 6: Clampless generative and browinsh skeletal hyphae of Phellinus gilvus (Schw.) Pat. X40. KOH 5% + Phloxin stained slide. Figure 7: Photomicrograph of hymenial setae of Phellinus palmicola (Berk. & Curt.) Ryv. X40. KOH 5% + Phloxin stained slide. Figure 8: Scanning electron photomicrograph of hooked and straight hymenial setae of Phellinus wahlbergii (Fr.) D.A. Reid. X500. Figures 5, 6, 7 © Aristóteles Góes-Neto. Figure 8 © Cláudia Groposo.

Clockwise from top left: Figure 9: Ressupinate basidiome of Phellinus membranaceus J.E. Wright & Blumenf. © Aristóteles Góes-Neto. Figure 10: Effuse-reflexed basidiomata of Phellinus gilvus (Schw.) Pat. © Fred Stevens, courtesy MykoWeb. Figure 11: Pileate basidiome of Phellinus tremulae. © Dave Powell, USDA Forest Service, courtesy InsectImages.org (#0976062). Figure 12: Stipitate basidiome of Stipitochaete damicornis (Link) Ryvarden. © Cláudia Groposo.
Hymenochaetoid fungi exhibit almost all possible forms of basidiomata. Ressupinate (Fig. 9), effuse-reflex (Fig. 10) and pileate (Fig. 11) forms predominate, but stipitate (Fig. 12) forms also occur. The same is true for hymenophores, which can be smooth (Fig. 13), toothed (Fig. 14), poroid (Fig. 15), and even lamelate (Fig. 16) (Parmasto, 2001; Goes-Neto et al., 2001). They exhibit all the three main patterns of hyphal systems (mono, di and trimitic), and sterile elements (other than setoid structures) of the hymenium are often found in non-hymenochaetacean species (Hibbett and Thorne, 2001).

Clockwise from top left: Figure 13: Smooth hymenophore of Hymenochaete sp. © Heino Lepp, courtesy Australian National Botanic Gardens. Figure 14: Toothed hymenophore of Hydnochaete sp. © Cláudia Groposo. Figure 15: Poroid hymenophore of Phellinus igniarius (L.) Quél. © Marek Snowarski. Figure 16: Lamelate hymenophore of Rickenella swartzii (Fr.) Kuyper. © Mirek Junek
Most of the members of the hymenochaetoid clade predominantly consist of saprotrophic, lignicolous, white-rot species, although mycorrhizal, forming both ectomycorrhiza (Danielson, 1984), and orchid mycorrhyza (Umata, 1995), and even agaricoid bryophyte-associated species (Moncalvo et al., 2002; Redhead et al., 2002) are also found in this clade.
Although they are not restricted to the hymenochaetoid clade, styrylpyrones are found in all studied hymenochatacean members of the clade (Fiasson, 1982), which, together with other phenolic compounds, confer the typical xanthocroic reaction.
Phylogenetic Position of the Hymenochaetoid Clade
Contrary to most of the Homobasidiomycetes, the hymenochaetoid clade, as well as the cantharelloid and gomphoid-phalloid clades, include species with imperforate parenthesomes (Hibbett and Thorn 2001, Binder and Hibbett 2002). As this character is also encountered in non-homobasidiomycetous Hymenomycetes (e. g. Auriculariales and Dacrymycetales), one can argue that the presence of imperforate parenthesomes is the plesiomorphic condition in the Homobasidiomycetes, and this point of view would be in accordance with the basal position of the hymenochaetoid clade in homobasidiomycete phylogeny. Hibbett and Thorn (2001), however, suggested that this character could be potentially homoplasic, because of the apparent co-occurrence of imperforate and perforate parenthosomes in the polyporoid clade (Keller, 1997), which would be in conformity with a non-basal position of this clade. Therefore, the position of the hymenochaetoid clade in homomobasidiomycete phylogeny still is controversial (Binder and Hibbett, 2002).
Discussion of Phylogenetic Relationships
Relationships among putative hymenochaetoid clades are not well resolved. According to Wagner and Fisher (2002a), there is at least some support for one less inclusive clade comprising most of the "typical hymenochaetaceae" (Inonotus s.s., Phylloporia, Fulvifomes, Inocutis, Fomitiporella, Aurificaria, Phellinus s.s., Pseudoinonotus, Fomitiporia, Porodaedalea, Onnia, Mensularia, and Pseudochaete). This group is in accordance with the classic concept of the Hymenochaetales sensu Oberwinkler (1977), and the taxa exhibit a holocoenocytic behavior of the somatic mycelium (Wagner and Fischer, 2001, 2002a), but its monophyly is still uncertain.
The remaining hymenochaetacean taxa (Coltricia, Coltriciella, Pyrrhoderma, Fuscoporia, Phellinidium, Asterodon, Phellopilus, Hymenochaete) appear intermingled with corticiaceous and polyporoid species of the genera Hyphodontia, Schizopora, Basidioradulum, Oxyporus, Bridgeoporus and Trichaptum, and, most surprisingly, also with gilled omphalinoid fungi, such as Cotylidia, Rickenella, Loreleia, Contumyces and Sphagnomphalia, as was revealed by previous studies (Hibbett and Donoghue, 1995, Hibbett et al. 1997, Ko et al., 1997, Hibbett et al., 2000; Hibbett and Donoghue, 2001; Moncalvo et al., 2002; Redberg et al., 2003; Redhead et al., 2002).
In summary, the position of the hymenochaetoid clade and the relationships among the putative more inclusive clades are definetely not well resolved. Thus, the great challenging tasks concerning this clade are (i) to discover which non-hymenochaetacean taxa are in this group and (ii) to characterize this clade more properly.
References
Ajith, T. A., Janardhanan, K. K. 2003. Cytotoxic and antitumor activities of a polypore macrofungus, Phellinus rimosus (Berk) Pilat. Journal of Ethnopharmacology 84: 157-162.
Binder, M.,Hibbett, D.S. 2002. Higher level phylogenetic relationships of homobasidiomycetes (mushroom-forming fungi) inferred from four rDNA regions. Mol. Phylogenet. Evol. 22: 76-90.
Burdsall, H. H.; Volk, T. J.; Ammirati, J. F. 1996. Bridgeoporus, a new genus to accommodate Oxyporus nobilissimus (Basidiomycota, Polyporaceae). Mycotaxon 60:387-395.
Danielson, R. M. 1984. Ectomycorrhizal associations in jack pine stands in northeastern Alberta. Can. J. Bot. 62:932-939.
Fiasson, J. L. 1982. Distribution of styrylpyrones in the basidiocarps of various Hymenochaetaceae. Biochemical Systematics and Ecology 10(4): 289-296.
Fischer, 2002
Góes-Neto, A.; Loguercio-Leite, C.; Guerrero, R. T., 2001d. Morphological Cladistic Analysis of Tropical Hymenochaetales (Basidiomycota). Mycotaxon 79: 467-479.
Harrington, T. C. and Wingfield, M. J. 1998. Diseases and the ecology of indigenous and exotic pines. Pp. 3-33 (chapter 19) in: Ecology and Biogeography of Pinus (D. M. Richardson, ed.). Cambridge University Press.
Hibbett, D. S., and Donoghue, M. J. 1995. Progress toward a phylogenetic classification of the Polyporaceae through parsimony analysis of mitochondrial ribosomal DNA sequences Can. J. Bot. 73(Suppl. 1):S583-S861.
Hibbett, D. S., Pine, E. M., Langer, E., Langer, G. and Donoghue, M. J. 1997. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc Nat Acad Sci USA 94:12002-12006.
Hibbett, D. S.; Gilbert, L-B.; Donoghue, M. J., 2000. Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature 407(6803): 506-508.
Hibbett, D. S.; Donoghue, M. J., 2001. Analysis of character correlations among wood decay mechanisms, mating systems, and substrate ranges in Homobasidiomycetes, Systematic Biology 50(2): 215-242.
Hibbett, D. S., and R. G. Thorn. 2001. Basidiomycota: Homobasidiomycetes. Pp. 121-168 in: The Mycota, vol. VII part B, Systematics and Evolution (D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke, eds.). Springer Verlag.
Keller J. 1997. Atlas des Basidiomycetes vus aux Microscopes Electroniques. Neuchâtel, Suisse: Union des Societes Suisses de Mycologie. 173 p 324 pl.
Ko, K. S., Hong, S. G., Jung, H. S. 1997. Phylogenetic analysis of Trichaptum based on nuclear 18S, 5.8S and ITS ribosomal DNA sequences. Mycologia 89: 727-734.
Larsen, M. J.; Cobb-Poule, L. A., 1990. The Genus Phellinus (Hymenochaetaceae): a survey of the world taxa. Synopsis. Fungorum 3: 1- 206.
Langer, E.; Oberwinkler, F., 1993. Corticioid basidiomycetes. I. Morphology and ultrastructure. Windahlia, 20: 1-28.
Lim, Y. W., Lee, J. S., Jung, H. S. 2003. Type studies on Phellinus baumii and Phellinus linteus. Mycotaxon 85: 201-210.
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, Miller OK Jr. 2002. One Hundred and Seventeen Clades of Euagarics. Mol Phyl Evol 23: 357-400.
Moore, R. T. 1985. The challenge of the dolipore/parenthesome septum. Pp. 175-212, in: D. Moore, L. A. Casselton, D. A. Wood, and J. Frankland (eds.) Developmental biology of higher fungi. Cambridge University Press, Cambridge.
Muller, W. H.; Stalpers, J. A.; van Aelst, A. C.; de Jong, M. D. M.; van der Krift, T. P.; Boekhout, T., 2000. The taxonomic position of Asterodon, Asterostroma and Coltricia inferred from the septal pore cap ultrastructure. Mycological Research 104(12): 1485-1491.
Oberwinkler, F. 1985. Anmerkungen zur Evolution und Systematik der Basidiomyceten. Studien an Heterobasidiomyceten, Teil 49. Bot Jahrb Syst 107(1-4):541-580.
Oberwinkler, F. 1977. Das neue System der Basidiomyceten. In Beiträge zur Biologie der Niederen Pflanzen (H. Frey, H. Hurka, and F. Oberwinkler, Eds.), pp 59-105, G. Fischer, Stuttgart, Germany.
Parmasto, Erast. 2001. Hymenochaetoid fungi (Basidiomycota) of North America. Mycotaxon 79: 107-176.
Redberg, G. L., R. Rodriguez, J. Ammirati, and D. S. Hibbett. 2003. Phylogenetic relationships of Bridgeoporus nobilissimus inferred from rDNA sequences. Mycologia 95: 685-687.
Redhead, Scott A., Jean-Marc Moncalvo, Rytas Vilgalys & François Lutzoni. Phylogeny of agarics: partial systematics solutions for bryophilous omphalinoid agarics outside of the Agaricales (euagarics), Mycotaxon 82: 151-168, 2002.
Ryvarden, L. 1991. Genera of Polypores: Nomenclature and Taxonomy. Synopsis. Fungorum. 5: 1-363.
Ryvarden, L. 1993. Tropical Polypores. In: Aspects of Tropical Mycology (S. Isaac, J. C. Frankland, R. Watling, A. J. S. Whalley, Eds.), pp. 149-170, Cambridge University Press, Cambridge.
Shon, M. Y., Kim, T. H., Sung, N. J. 2003. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chemistry 82: 593-597.
Thies, W. G. and Sturrock, R. N. 1995. Laminated root rot in western North America. USDA For. Serv., CFS Res. Bull. PNW GTR 349.
Umata, H. 1995. Seed germination of Galeola altissima, an achlorophyllous orchid, with aphyllophorales fungi. Mycoscience 36: 368-372.
Wagner T. and Fischer M. 2001. Natural goups and a revised systemfor the European poroid Hymenochaetales (Basidiomycota) supported by nLSU rDNA sequence data. Mycological Research 105(7): 773-782.
Wagner T. and Fischer M. 2002a. Proceedings towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l., and phylogenetic relationships of allied genera. Mycologia 94: 998-1016.
Wagner, T. and Fischer, M. 2002b. Classification and phylogenetic relationships of Hymenochaete and allied genera of the Hymenochaetales, inferred from rDNA sequence data and nuclear behaviour of vegetative mycelium. Mycological Progress 1(1): 93-104.
Wagner, T. and Ryvarden, L. 2002. Phylogeny and taxonomy of the genus Phylloporia (Hymenochaetales). Mycological Progress 1(1): 105-116.
Wesenberg, D., Kyriakides, I. and Agathos, S.N. 2003. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Adv. 22(1-2):161-187.
Information on the Internet
- The WWW Virtual Library: mycology. An index to Mycological Resources in the internet.
- Deep Hypha Research Coordination Network. Deep Hypha is a project to coordinate and provide resources for research in fungal systematics.
- AFTOL: Assembling the Fungal Tree of Life. Collaborative research in fungal phylogenetics.
Title Illustrations

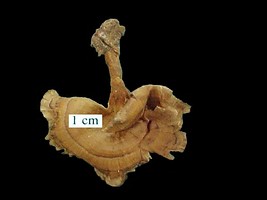
| Scientific Name | Aurificaria luteoumbrina (Romell) D.A. Reid |
|---|---|
| Location | Brazil, Bahia, Cachoeira |
| Specimen Condition | Live Specimen |
| Identified By | Aristóteles Góes-Neto |
| Body Part | basidiomata |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Aristóteles Góes-Neto

|
About This Page
Aristóteles Góes-Neto

Universidade Estadual de Feira de Santana (UEFS), Brazil
Cláudia Groposo

Universidade Estadual de Feira de Santana (UEFS), Brazil
Correspondence regarding this page should be directed to Aristóteles Góes-Neto at and Cláudia Groposo at
Page copyright © 2004 Aristóteles Góes-Neto and Cláudia Groposo
 Page: Tree of Life
Hymenochaetales. Hymenochaetoid clade. Hymenochaetaceae.
Authored by
Aristóteles Góes-Neto and Cláudia Groposo.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Hymenochaetales. Hymenochaetoid clade. Hymenochaetaceae.
Authored by
Aristóteles Góes-Neto and Cláudia Groposo.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 10 June 2005
Citing this page:
Góes-Neto, Aristóteles and Cláudia Groposo. 2005. Hymenochaetales. Hymenochaetoid clade. Hymenochaetaceae. Version 10 June 2005. http://tolweb.org/Hymenochaetoid_clade/20547/2005.06.10 in The Tree of Life Web Project, http://tolweb.org/






















 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site