magnoliids
Pam Soltis, Doug Soltis, and Christine Edwards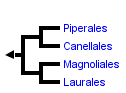

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The magnoliid clade contains several thousand (~8500) species divided into 20 families, including several large, economically important families such as Magnoliaceae, Lauraceae, Piperaceae, and Annonaceae. Members of the magnoliid clade are widespread throughout tropical and temperate areas of the world and can be large trees, shrubs, vines, lianas, and occasionally herbs (Judd et al., 2002; D. Soltis et al., 2005). Economically important products derived from magnoliids include edible fruits, such as avocados (Persea americana) and guanabana, sour sop, cherimoya, and sweet sop (Annona spp.). Magnoliid species are also the source of spices (Judd et al., 2002; D. Soltis et al., 2005) such as black and white pepper (Piper nigrum), bay leaves (Laurus nigrus), nutmeg (Myristica fragrans), cinnamon (Cinnamomum verum), and camphor (Cinnamomum camphora). Other members are used as ornamentals or timber (e.g., Magnolia and Liriodendron; Judd et al., 2002; D. Soltis et al., 2005).
Characteristics
The magnoliid clade contains most of those lineages that were typically referred to as "primitive angiosperms" in earlier classification schemes (e.g., Cronquist, 1981, 1988; Stebbins, 1974; Takhtajan, 1997); most of these "primitive" species have long, broad, net-veined leaves and large flowers with numerous, spirally arranged tepals (perianth parts that are not differentiated into petals and sepals), stamens, and carpels. Some also have leaf-like, or "laminar," stamens. However, while these classification systems (for example Cronquist's subclass Magnoliidae) united some of the families that are currently circumscribed as magnoliids, phylogenetic analyses have shown that these previous classifications also included groups that are unrelated to the magnoliid clade, such as Amborella and Austrobaileyales. Moreover, these classifications also excluded members, such as Lactoris, that were difficult to place in the magnoliids because the group is very old (some hypothesized members, such as Archaeanthus (Dilcher and Crane 1984) date to the early Cretaceous) and extinction has likely obscured many of the evolutionary relationships (see Soltis et al 2005).
The major lineages of magnoliids were first identified in studies using DNA evidence (e.g., P. Soltis et al., 1999); however the composition of the magnoliid clade and relationships among the members did not become clear until data sets of at least five genes for a broad sample of taxa were assembled to address these problems (e.g., Qiu et al., 1999, 2000; Zanis et al., 2002). Several possible morphological characteristics have now been identified that may unite magnoliids. Some (although obscure) possible synapomorphies (shared, derived characteristics) include entire leaf margins, extrorse anthers, ephemeral antipodal cells, the presence of hypostase, the presence of a nucellar cap, and raphal bundle branches at the chalaza (Angiosperm Phylogeny Website, 2005).
Discussion of Phylogenetic Relationships
Within the magnoliids, researchers have identified four evolutionary lineages and clarified their evolutionary relationships: Magnoliales and Laurales are sisters, and Piperales and Canellales are sisters. The circumscription of these orders and their interrelationships had not been proposed previously based on morphology alone.
Magnoliales. Based on molecular analyses, this group includes six families (Myristicaceae, Degeneriaceae, Himantandraceae, Magnoliaceae, Eupomatiaceae, and Annonaceae), relationships among which are now clear (e.g., Sauquet et al., 2003). This same clade also emerged in the nonmolecular analysis of Doyle and Endress (2000). Apparent synapomorphies for the clade include reduced fiber pit borders, stratified phloem, an adaxial plate of vascular tissue in the petiole, palisade parenchyma, asterosclereids in the leaf mesophyll, continuous tectum in the pollen, and multiplicative testa in the seed (Doyle and Endress, 2000). Furthermore, all members of this clade examined to date have a characteristic deletion in their Apetala3 gene (Kim et al., 2004a).



Magnolia tripetala, a member of Magnoliaceae (photo © Kurt Stuber), and Eupomatia benettii, a representative of Epomatiaceae (photo © Peter Endress).
Laurales. Laurales, as currently circumscribed (APG II, 2003; see Renner, 1999), include seven families: Calycanthaceae (including Idiospermaceae), Monimiaceae, Gomortegaceae, Atherospermataceae, Lauraceae, Siparunaceae, and Hernandiaceae. In previous classifications (e.g., Cronquist, 1981, 1988), Amborellaceae and Trimeniaceae were occasionally placed in Laurales; and in fact, both Amborella and Trimenia have even been considered part of Monimiaceae (Perkins, 1925; see Angiosperms for a discussion of the placement of these families). Chloranthaceae have also occasionally been placed in Laurales (e.g., Takhtajan, 1987, 1997; Thorne, 1974; see Angiosperms for a discussion of its placement). Laurales are united by a perigynous flower in which the gynoecium is frequently deeply embedded in a fleshy receptacle (Endress and Igersheim, 1997; Renner, 1999). Other apparent non-DNA synapomorphies include the presence of inner staminodia, ascendant ovules, and tracheidal endotesta (Doyle and Endress, 2000).


Photo of Persea americana, a representative of Lauraceae (photo © Matyas Buzgo).
Piperales. Previous circumscriptions of Piperales have varied (e.g., Dahlgren, 1980; Cronquist, 1981, 1988; Takhtajan, 1987, 1997; Thorne, 1992; Heywood, 1993), but molecular studies clearly united Aristolochiaceae, Lactoridaceae, Piperaceae, and Saururaceae (e.g., Qiu et al., 1999; P. Soltis et al., 1999; Barkman et al., 2000; D. Soltis et al., 2000; Zanis et al., 2002). In addition, recent studies have placed Hydnoraceae, a family of parasitic plants often placed in Rosidae (e.g., Cronquist, 1981; Heywood, 1993), within Piperales, although the exact position is not certain (Nickrent et al., 2002). Although not recognized as a group prior to molecular analyses, a number of morphological synapomorphies have been identified: distichous phyllotaxis, a single prophyll, and oil cells (Doyle and Endress, 2000).



The flower of Aristolochia sprucei, a representative of Aristolochiaceae (photo © Mario Blanco), the inflorescence of Piper capense, in the Piperaceae (photo © Matyas Buzgo), and the inflorescence of Houttuynia cordata, a representative of Saururaceae (photo © Matyas Buzgo).
Canellales. The relationship of Canellaceae and Winteraceae has been strongly supported in all multigene analyses (e.g., Qiu et al., 1999; P. Soltis et al., 1999; D. Soltis et al., 2000; Zanis et al., 2002, 2003), and the clade was also obtained in Doyle and Endress's (2000) nonmolecular analysis. However, these two families have not typically been considered closely related to each other, and neither was suspected of being related to any members of Piperales. For example, Winteraceae have often been considered a close relative of Magnoliaceae (e.g., Cronquist, 1981, 1988; Heywood, 1993), with Canellaceae close to Myristicaceae (e.g., Wilson, 1966; Cronquist, 1981, 1988). Furthermore, intuitive classification systems regarded Winteraceae as perhaps the "most primitive" extant family of angiosperms (Cronquist, 1981; Endress, 1986). The phylogenetic position of Winteraceae clearly indicates that the vesselless xylem and plicate carpels found in members of the family are secondarily derived (see also Young, 1981). Possible synapomorphies for Canellales are a well-differentiated pollen tube transmitting tissue, an outer integument with only two to four cell layers, and seeds with a palisade exotesta (Doyle and Endress, 2000). Additional synapomorphies may include an irregular "first-rank" leaf venation (Hickey and Wolf, 1975; Doyle and Endress, 2000), features of stelar and nodal structure (Keating, 2000), and vascularization of the seeds (Deroin, 2000).
References
Angiosperm Phylogeny Group (APG). 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531-553.
Angiosperm Phylogeny Group (APG II). 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399-436.
Baillon, H. 1869. Histoires des plantes, vol. 1. L. Hachette & Cie. Paris, France.
Barkman, T. J., G. Chenery, J. R. Mcneal, J. Lyons-Weiler, and C. W. Depamphilis. 2000. Independent and combined analyses of sequences from all three genomic compartments converge on the root of flowering plant phylogeny. Proceedings of the National Academy of Sciences, USA 97: 13166-13171.
Basinger, J., and D. L. Dilcher. 1984. Ancient bisexual flowers. Science 224: 511-513.
Braun, A. 1864. Uebersicht der naturlichen Systems nach der Anordnung derselben. In P. Ascherson [ed.], Flora der Provinz Brandenburg, der Altmark und des Herzogthums Magdeburg, vol. 1, 22-67. Hirschwald, Berlin, Germany.
Carlquist, S. 1990. Wood anatomy and relationships of Lactoridaceae. American Journal of Botany 77:1498-1505.
Carlquist, S. 2000. Wood and bark anatomy of Takhtajania (Winteraceae); Phylogenetic and ecological implications. Annals of the Missouri Botanical Garden 87:317-322.
Chanderbali, A. S., H. van der Werff, and S. S. Renner. 2001. Phylogeny and historical biogeography of Lauraceae: Evidence from the chloroplast and nuclear genomes. Annals of the Missouri Botanical Garden 88:104-134.
Chase, M. W., and V. A. Albert. 1998. A perspective on the contribution of plastid rbcL DNA sequences to angiosperm phylogenetics. In D. E. Soltis, P. S. Soltis, and J. J. Doyle [eds.], Molecular systematics of plants, vol. 2: DNA sequencing, 488-507. Kluwer, Boston, Massachusetts, USA.
Chase, M. W., D. E. Soltis, R. G. Olmstead, D. Morgan, D. H. Les, B. D. Mishler, M. R. Duvall, R. A. Price, H. G. Hills, Y.-L. Qiu, K. A. Kron, J. H. Rettig, E. Conti, J. D. Palmer, J. R. Manhart, K. J. Sytsma, H. J. Michaels, W. J. Kress, K. G. Karol, W. D. Clark, M. Hedren, B. S. Gaut, R. K. Jansen, K.-J. Kim, C. F. Wimpee, J. F. Smith, G. R. Furnier, S. H. Strauss, Q.-Y. Xiang, G. M. Plunkett, P. S. Soltis, S. M. Swensen, S. E. Williams, P. A. Gadek, C. J. Quinn, L. E. Eguiarte, E. Golenberg, G. H. Learn, Jr., S. W. Graham, S. C. H. Barrett, S. Dayanandan, and V. A. Albert. 1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528-580.
Couper, R. A. 1958. British Mesozoic microspores and pollen grains: a systematic and stratigraphic study. Palaeontographica. Abteilung B, Paläophytologie 103: 75-179.
Crane, P. R., E. M. Friis, and K.R. Pedersen. 1995. The origin and early diversification of angiosperms. Nature 374: 27-33.
Cronquist, A. 1981. An integrated system of classification of flowering plants. Columbia University Press, New York, USA.
Cronquist, A. 1988. The evolution and classification of flowering plants, 2nd ed. New York Botanical Garden, Bronx, New York, USA.
Dahlgren, R. 1980. A revised system of classification of the angiosperms. Botanical Journal of the Linnean Society 80: 91-124.
Davies, T. J., T. G. Barraclough, M. W. Chase, P. S. Soltis, D. E. Soltis, And V. Savolainen. 2004. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences, USA 101: 1904-1909.
De Jussieu, A. L. 1789. Genera plantarum secundum ordines naturals disposita. Heissant and Barrois, Paris, France.
Deroin, T. 2000. Notes on the vascular anatomy of the fruit of Takhtajania (Winteraceae) and its interpretation. Annals of the Missouri Botanical Garden 87: 398-406.
Dilcher, D. L., and Crane, P. R. 1984. Archaeanthus: an early angiosperm from the Cenomanian of the western interior of North America. Annals of the Missouri Botanical Garden 71: 351-383.
Donoghue, M. J., and J. A. Doyle. 1989. Phylogenetic studies of seed plants and angiosperms based on morphological characters. In K. Bremer and H. Jörnvall [eds.], The hierarchy of life: molecules and morphology in phylogenetic studies, 181-193. Elsevier Science Publishers, Amsterdam, The Netherlands.
Doust, A. N. 2000. Comparative floral ontogeny in Winteraceae. Annals of the Missouri Botanical Garden 87:366-379.
Doyle, J. A. 2000. Paleobotany, relationships, and geographic history of Winteraceae. Annals of the Missouri Botanical Garden 87: 303-316.
Doyle, J. A., and M. J. Donoghue. 1986. Seed plant phylogeny and the origin of the angiosperms: an experimental cladistic approach. Botanical Review 52: 321-431.
Doyle, J. A., and M. J. Donoghue. 1992. Fossils and seed plant phylogeny reanalzyed. Brittonia 44: 89-106.
Doyle, J. A., M. J. Donoghue and E. A. Zimmer. 1994. Integration of morphological and rRNA data on the origin of angiosperms. Annals of the Missouri Botanical Garden 81:419-450.
Doyle, J. A., C. L. Hotton and J. V. Ward. 1990. Early Cretaceous tetrads, zonasuculate pollen and Winteraeae. I. Taxonomy, morphology, and ultrastructure. American Journal of Botany 77:1544-1557.
Doyle, J. A., C. L. Hotton and J. V. Ward. 1990. Early Cretaceous tetrads, zonasuculate pollen, and Winteraceae. II. Cladistic analysis and implications. American Journal of Botany 77:1558-1568.
Doyle, J. A., and P. K. Endress. 2000. Morphological phylogenetic analyses of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences 161 (Supplement): S121-S153.
Drinnan, A. N., P. R. Crane, E. M. Friis, and K. R. Pedersen. 1990. Lauraceous flowers from the Potomac group (mid-Cretaceous) of eastern North America. Botanical Gazette 151:370-384.
Ehrendorfer, F. and M. Lambrou. 2000. Chromosomes of Takhtajania, other Winteraceae, and Canellaceae: Phylogenetic implications. Annals of the Missouri Botanical Garden 87:407-413.
Eklund, H. 2000. Lauraceous flowers from the Late Cretaceous of North Carolina, USA. Botanical Journal of the Linnean Society 132:397-428.
Endress, P. K. 1984. The flowering process in the Eupomatiaceae (Magnoliales). Bot. Jahrb. Syst. 104:297-319.
Endress, P. K. 1986. Reproductive structures and phylogenetic significance of extant primitive angiosperms. Plant Systematic Evolution 152: 1-28.
Endress, P. K. 2001. The flowers in extant basal angiosperms and inferences on ancestral flowers. International Journal of Plant Sciences 162: 1111-1140.
Endress, P. K., and A. Igersheim. 1997. Gynoecium diversity and systematics of the Laurales. Botanical Journal of the Linnean Society 125: 93-168.
Endress, P. K., and A. Igersheim. 2000a. The reproductive structures of the basal angiosperm Amborella trichopoda (Amborellaceae). International Journal of Plant Sciences 161 (Supplement): S237-S248.
Endress, P. K., and A. Igersheim. 2000b. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences 161 (Supplement): S211-S223.
Endress, P. K., A. Igersheim, F. B. Sampson, and G. E. Schatz. 2000. Floral structure of Takhtajania and its systematic position in Winteraceae. Annals of the Missouri Botanical Garden 87:347-365.
Feild, T. S., T. Brodribb, and M. Holbrook. 2002. Hardly a relict: Freezing and the evolution of vesselless wood in Winteraceae. Evolution 56:464-478.
Feild, T. S., M. A. Zwieniecki, and M. Holbrook. 2000. Winteraceae evolution: An ecophysiological perspective. Annals of the Missouri Botanical Garden 87:323-334.
Friis, E. M., K. R. Pedersen, and P. R. Crane. 2000. Reproductive structure and organization of basal angiosperms from the early Cretaceous (Barremian or Aptian) of western Portugal. International Journal of Plant Sciences 161 (Supplement): S169-S182.
Gandolfo, M. A., K. C. Nixon, and W. L. Crepet. 2002. Triuridaceae fossil flowers from the Upper Cretaceous of New Jersey. American Journal of Botany 89: 1940-1957.
Gandolfo, M. A., K. C. Nixon, and W. L. Crepet. 2004. Cretaceous flowers of Nymphaeaceae and implications for complex insect entrapment pollinations mechanisms in early Angiosperms. Proceedings of the National Academy of Sciences, USA: online.
Gonzalez, F. and P. Rudall. 2001. The questionable affinities of Lactoris: Evidence from branching pattern, inflorescence morphology, and stipule development. American Journal of Botany 88:2143-2150.
Gottsberger, G. 1988. The reproductive biology of primitive angiosperms. Taxon 37: 630-643.
Graham, S. W., and R. G. Olmstead. 2000. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. American Journal of Botany 87: 1712-1730.
Heo, K., H. van der Werff, and H. Tobe. 1998. Embryology and relationships of Lauraceae (Laurales). Botanical Journal of the Linnean Society 126:295-322.
Heywood, V. 1993. Flowering plants of the world. B.T. Batsford Ltd., London, UK.
Hickey, L. J., and A. D. Wolfe. 1975. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden 62: 538-589.
Hilu, K. W., T. Borsch, K. Muller, D. E. Soltis, P. S. Soltis, V. Savolainen, M. Chase, M. Powell, L. Alice, R. Evans, H. Sauquet, C. Neinhuis, T. Slotta, J. Rohwer, and L. Chatrou. 2003. Inference of angiosperm phylogeny based on matK sequence information. American Journal of Botany 90: 1758-1776.
Hoot, S. B., S. Magallón, and P. R. Crane. 1999. Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Annals of the Missouri Botanical Garden 86: 1-32.
Igersheim, A. and P. K. Endress. 1998. Gynoecium diversity and systematics of the paleoherbs. Botanical Journal of the Linnean Society 127:289-370.
Judd, W. S., C. S. Campbell, E. A. Kellogg, P. F. Stevens, and M. J. Donoghue. 2002. Plant systematics: a phylogenetic approach. Sinauer Associates, Inc., Sunderland, Massachusetts, USA.
Karol, K. G., Y. B. Suh, G. E. Schatz, and E. A. Zimmer. 2000. Molecular evidence for the phylogenetic position of Takhtajania in the Winteraceae: Inference from nuclear ribosomal and chloroplast gene spacer sequences. Annals of the Missouri Botanical Garden 87:414-432.
Keating, R. C. 2000. Anatomy of the young vegetative shoot of Takhtajania perrieri (Winteraceae). Annals of the Missouri Botanical Garden 87: 335-346.
Kim, S., V. A. Albert, M.-J. Yoo, J. S. Farris, M. Zanis, P. S. Soltis, and D. E. Soltis. 2004a. Pre-angiosperm duplication of floral genes and regulatory tinkering at the base of flowering plants. American Journal of Botany: in press.
Kimoto, Y. and H. Tobe. 2001. Embryology of Laurales: a review and perspectives. Journal of Plant Research 114:247-267.
Kramer, E. M., R. L. Dorit, and V. F. Irish. 1998. Molecular evolution of genes controlling petal and stamen development: Duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149: 765-783.
Litt, A., and V. Irish. 2003. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165: 821-833.
Loconte, H., and D. W. Stevenson. 1991. Cladistics of the Magnoliidae. Cladistics 7: 267-296.
Mathews, S., and M. J. Donoghue. 1999. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286: 947-949.
Mathews, S., and M. J. Donoghue. 2000. Basal angiosperm phylogeny inferred from duplicate phytochromes A and C. International Journal of Plant Sciences 161 (Supplement): S41-S55.
Nandi, O. I., M. W. Chase, and P. K. Endress. 1998. A combined cladistic analysis of angiosperms using rbcL and nonmolecular data sets. Annals of the Missouri Botanical Garden 85: 137 - 212.
Nickrent, D. L., A. Blarer, Y.-L. Qiu, D. E. Soltis, P. S. Soltis, and M. Zanis. 2002. Molecular data place Hydnoraceae with Aristolochiaceae. American Journal of Botany 89: 1809-1817.
Nickerson, J., and G. Drouin. 2004. The sequence of the largest subunit of RNA polymerase II is a useful marker for inferring seed plant phylogeny. Molecular Phylogenetics and Evolution 31: 403-415.
Parkinson, C. L., K. L. Adams, and J. D. Palmer. 1999. Multigene analyses identify the three earliest lineages of extant flowering plants. Current Biology 9: 1485-1488.
Perkins, J. 1925. Ubersicht uber die Gattungen der Monimiaceae. Engelmann, Leipzig, Germany.
Qiu, Y. L., M. W. Chase, D. H. Les and C. R. Parks. 1993. Molecular phylogenetics of the Magnoliidae: cladistic analyses of nucleotide sequences of the plastid gene rbcL. Ann. Missouri Bot. Gard. 80:587-606.
Qiu, Y.-L., J. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zanis, Z. Chen, V. Savolainen, and M. W. Chase. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404-407.
Qiu, Y.-L., J.-Y. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zanis, E. Zimmer, Z. Chen, V. Savolainen, and M. Chase. 2000. Phylogeny of basal angiosperms: analyses of five genes from three genomes. International Journal of Plant Sciences 161 (Supplement): S3-S27.
Ray, J. 1703. Methodus plantarum emendata et aucta. Smith and Walford, London, UK.
Reichenbach, H. G. L. 1827-1829. Dr. Joh. Christ, Moessler's Handbuch der Gewaechskunde, [ed.] vols. 2, 3. Hammerich, Altona, Germany.
Renner, S. S. 1999. Circumscription and phylogeny of the Laurales: evidence from molecular and morphological data. American Journal of Botany 86: 1301-1315.
Renner, S. S. and A. S. Chanderbali. 2000. What is the relationship among Hernandiaceae, Lauraceae, and Monimiaceae, and why is this question so difficult to answer? International Journal of Plant Sciences 161:S109-S119.
Renner, S. S., A. E. Schwarzbach, and L. Lohmann. 1997. Phylogenetic position and floral function of Siparuna (Siparunaceae: Laurales). International Journal of Plant Sciences 158:S89-S98.
Ronse De Craene, L. P., P. S. Soltis, and D. E. Soltis. 2003. Evolution of floral structures in basal angiosperms. International Journal of Plant Sciences 164 (Supplement): S329-S363.
Sampson, F. B. 2000. The pollen of Takhtajania perrieri (Winteraceae). Annals of the Missouri Botanical Garden 87:380-388.
Sauquet, H., J. A. Doyle, T. Scharaschkin, T. Borsch, K. W. Hilu, L. W. Chatrou, and A. Le Thomas. 2003. Phylogenetic analysis of Magnoliales and Myristicaceae based on multiple data sets: implications for character evolution. Botanical Journal of the Linnean Society 142: 125-186.
Schatz, G. E., P. P. Lowry, and A. Ramisamihantanirina. 1998. Takhtajania perrieri rediscovered. Nature 391:133-134.
Soltis, D. E., P. S. Soltis, D. L. Nickrent, L. A. Johnson, W. J. Hahn, S. B. Hoot, J. A. Sweere, R. K. Kuzoff, K. A. Kron, M. W. Chase, S. M. Swensen, E. A. Zimmer, S.-M. Chaw, L. J. Gillespie, W. J. Kress, and K. J. Sytsma. 1997. Angiosperm phylogeny inferred from 18S ribosomal DNA sequences. Annals of the Missouri Botanical Garden 84: 1-49.
Soltis, D. E., P. S. Soltis, M. E. Mort, M. W. Chase, V. Savolainen, S. B. Hoot, and C. M. Morton. 1998. Infering complex phylogeneis using parsimony: an empirical approach using three large DNA data sets for angiosperms. Systematic Biology 47: 32-42.
Soltis, D. E., P. S. Soltis, M. W. Chase, M. E. Mort, D. C. Albach, M. Zanis, V. Savolainen, W. J. Hahn, S. B. Hoot, M. F. Fay, M. Axtell, S. M. Swensen, L. M. Prince, W. J. Kress, K. C. Nixon, and J. S. Farris. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381-461.
Soltis, D. E., P. S. Soltis, M. W. Chase, and P. K. Endress. 2005. Angiosperm phylogeny and evolution. Sunderland: Sinauer Associates
Soltis, P. S., D. E. Soltis, and M. W. Chase. 1999. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402:402-404.
Soltis, P. S., D. E. Soltis, M. J. Zanis, and S. Kim. 2000. Basal lineages of angiosperms: Relationships and implications for floral evolution. International Journal of Plant Science161(Supplement): S97-S107.
Soltis, P. S., D. E. soltis, V. savolainen, P. R. Crane, and T. G. Barraclough. 2002. Rate heterogeneity among lineages of tracheophytes: integration of molecular and fossil data and evidence for molecular living fossils. Proceedings of the National Acadamy of Sciences, USA 99: 4430-4435.
Soltis, P. S., D. E. Soltis, M. W. Chase, P. K. Endress, and P. R. Crane. 2004. The diversification of flowering plants. In J. Cracraft and M. J. Donoghue [eds.], The tree of life. Oxford University Press, Oxford, UK.
Sytsma, K. J., and D. A. Baum. 1996. Molecular phylogenies and the diversification of angiosperms. In D. W. Taylor and L. J. Hickey [eds.], Flowering plant origin, evolution, and phylogeny, 314-340. Chapman and Hall, New York, USA.
Suh, Y., L. B. Thien, H. E. Reeve and E. A. Zimmer. 1993. Molecular evolution and phylogenetic implications of internal transcribed spacer sequences of ribosomal DNA Winteraceae. American Journal of Botany 80:1042-1055.
Takhtajan, A. 1980. Outline of the classification of flowering plants (Magnoliophyta). Botanical Review 46: 225-359.
Takhtajan, A. 1987. System of Magnoliophyta. Academy of Sciences, Leningrad, USSR.
Takhtajan, A. 1997. Diversity and classification of flowering plants. Columbia University Press, New York, USA.
Thorne, R. F. 1974. A phylogenetic classification of the Annoniflorae. Aliso 8: 147-209.
Thorne, R. F. 1992. Classification and geography of the flowering plants. Botanical Review 58: 225-348.
Tobe, H. and B. Sampson. 2000. Embryology of Takhtajania (Winteraceae) and a summary statement of embryological features for the family. Annals of the Missouri Botanical Garden 87:389-397.
Tucker, S. C., A. W. Douglas, and L. Han-Xing. 1993. Utility of Ontogenetic and Conventional Characters in Determining Phylogenetic Relationships of Saururaceae and Piperaceae (Piperales). Systematic Botany 18:614-641.
Van der Ham, R., and B. J. Van Heuven. 2002. Evolutionary trends in Winteraceae pollen. Grana 41:4-9.
Vink, W. 1988. Taxonomy in Winteraceae. Taxon 37:691-698.
Vink, W. 1993. Winteraceae. Pages 630-638 in: The Families and Genera of Vascular Plants. II. Flowering Plants: Dicotyledons, Magnoliid, Hamamelid and Caryophyllid Families. K. Kubitzki, J. G. Rohwer, and V. Bittrich, eds. Springer, Berlin.
Walker, J. W., G. J. Breener, and A. G. Walker. 1983. Winteraceaeous pollen in the Lower Cretaceous of Israel: early evidence of a magnolialean angiosperm family. Science 220:1273-1275.
Walker, J. W., and A. G. Walker. 1984. Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants. Annals of the Missouri Botanical Garden 71: 464-521.
Wilson, T. K. 1966. The comparative morphology of the Canellaceae. IV. Floral morphology and conclusions. American Journal of Botany 53: 336-343.
Zanis, M., D. E. Soltis, P. S. Soltis, S. Mathews, and M. J. Donoghue. 2002. The root of the angiosperms revisited. Proceedings of the National Academy of Sciences, USA 99: 6848-6853.
Zanis, M. J., P. S. Soltis, Y.-L. Qiu, E. Zimmer, and D. E. Soltis. 2003. Phylogenetic analyses and perianth evolution in basal angiosperms. Annals of the Missouri Botanical Garden 90: 129-150.
Information on the Internet
- Angiosperm Phylogeny Website. P. F. Stevens, Missouri Botanical Garden.
- Deep Time Project: A Comprehensive Phylogenetic Tree of Living and Fossil Angiosperms. Florida Museum of Natural History.
- Treezilla (500-taxon rbcL Analyses). Ken Rice, Michael Donoghue, Dick Olmstead
- The Families of Flowering Plants. L. Watson and M. J. Dallwitz.
- Images and Descriptions of Flowering Plant Families (as treated by Arthur Cronquist). University of Hawaii Botany Department.
- Flowering Plant Gateway. Hugh Wilson, Texas A&M Bioinformatics Working Group.
- Classification of Flowering Plants. Kåre Bremer, Birgitta Bremer & Mats Thulin, Uppsala University.
- Thonner's analytical key to the families of flowering plants
- The Virtual Flower. Floral Genome Project (FGP).
- The Myristicaceae Pages. John Janovic, Manoj Mistry, and Roosevelt García Villacorta.
Title Illustrations

| Scientific Name | Aristolochia trilobata |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Mario Blanco |
| Body Part | Flower |
| View | profile |
| Copyright | © 2005 |
| Scientific Name | Tasmannia stipitata |
|---|---|
| Specimen Condition | Live Specimen |
| Identified By | Matyas Buzgo |
| Sex | Female |
| Body Part | Flowers |
| Copyright | © 2005 Matyas Buzgo |
About This Page

Florida Museum of Natural History and the Genetics Institute, Gainesville, Florida, USA
Doug Soltis

Department of Botany and the Genetics Institute, Gainesville, Florida, USA

Department of Botany, University of Wyoming, Laramie, Wyoming, USA
Correspondence regarding this page should be directed to Christine Edwards at
Page copyright © 2005 , , and
 Page: Tree of Life
magnoliids.
Authored by
Pam Soltis, Doug Soltis, and Christine Edwards.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
magnoliids.
Authored by
Pam Soltis, Doug Soltis, and Christine Edwards.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 23 June 2005
Citing this page:
Soltis, Pam, Doug Soltis, and Christine Edwards. 2005. magnoliids. Version 23 June 2005. http://tolweb.org/magnoliids/20670/2005.06.23 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site